About our research
We are studying atmospheric environment, safety and security, and advanced measurement technology from the standpoints of physical chemistry, reaction engineering, and spectroscopy.
Estimating the amount of greenhouse gases from natural sources
The amount of greenhouse gases produced by peatlands and forest fires is thought to be comparable to the amount produced by anthropogenic sources, but the details are unknown. We are developing a method for estimating the amount of naturally occurring greenhouse gases based on field surveys in peatlands and wide-area carbon dioxide observations.
Stable isotope measurements in greenhouse gases
By measuring the stable isotope ratios of greenhouse gases, it is possible to identify the source of the gases and investigate the material cycle in the atmosphere. Conventional methods have problems such as lack of real-time measurement in the field and complicated pretreatment of analysis samples. In this laboratory, we have successfully developed a near-infrared laser-based system for real-time stable isotope measurement in carbon dioxide, and are analyzing the environmental dynamics of carbon dioxide using this system.
Measurement of automobile exhaust gas
We are measuring trace gas components emitted from automobiles in collaboration with the National Institute for Traffic Safety and Environment using equipment developed by the following laboratories.
Development of an Atmospheric Nitrogen Oxide Concentration Measurement System Using a Mid-Infrared Quantum Cascade Laser
Nitrogen oxides (NOx) such as NO and NO2 in the atmosphere are attracting attention as substances that cause environmental problems such as photochemical smog and acid rain, even though they are trace gases at the ppb level, and highly sensitive atmospheric concentration measurement of NOx is required.
In this research, we are developing a measurement system using absorption spectroscopy with a quantum cascade laser at mid-infrared wavelengths. When light is passed through a material, a part of the light is absorbed, and the amount of absorption is proportional to the concentration of the absorbing material and the optical path length (Lambert-Beer law). Therefore, the concentration of the target substance can be determined by measuring the amount of light absorbed, and the measurement sensitivity of the device can be increased by increasing the optical path length to increase the absorption. In order to secure a long optical path in a small apparatus, high reflectivity mirrors are installed at both ends of the cell to make the light flow back and forth in the cell.
Fig. 1 shows a schematic diagram of the experimental apparatus. A laser beam is passed through the cell while gas is flowing inside, and the light coming out of the cell is detected. By measuring the intensity of the detected light with and without NOx in the cell, we can determine the amount of light absorbed by NO/NO2.
We have successfully detected NOx at the ppb level using the cavity ring-down spectroscopy (CRDS) method (Photo: Fig. 2), which has an optical path length of several kilometers. We will evaluate the performance of the device by measuring automobile exhaust gas using a chassis dynamometer, and apply it to atmospheric measurement.
Development of a portable photoionization time-of-flight mass spectrometer for measurement of volatile organic compounds in the atmosphere
Volatile organic compounds (VOCs) in the troposphere play a major role in the formation of suspended particulate matter and photochemical oxidants, and understanding their composition and concentration is important for understanding tropospheric atmospheric chemistry.
Therefore, we are developing a portable analyzer combining vacuum ultraviolet-photon ionization and time-of-flight mass spectrometry for real-time measurement of VOCs in the atmosphere.
“The vacuum ultraviolet-photon ionization (VUV-photon ionization) method is a soft ionization method that suppresses the excess energy of ionization (soft ionization), thereby suppressing the formation of fragment ions. 118 nm (10.5 eV) Xe third harmonic generation from the third harmonic of the Nd:YAG laser (355 nm) (10.5 eV) vacuum ultraviolet light is used for ionization.
“Time-of-flight mass spectrometry has the advantage of high ion transmission and the ability to acquire the entire mass spectrum in a single measurement. In addition, by using a reflector (electrostatic mirror) to increase the flight distance, we can improve the mass resolution and save space. The instrument developed in this laboratory (photo) has a mass resolution of 800 (m/z = 112), and the detection limit of VOCs is several ppbv (integrated for 5 seconds).
Mid-infrared absorption spectroscopic study on the detection and reaction of Creagie intermediates

He-Ne laser spot
The HOX cycle in the troposphere and stratosphere plays an important role in the formation and loss of ozone and the oxidation of volatile organic compounds (VOCs) in the atmosphere. Therefore, understanding the reaction mechanism of the HOX cycle is a very important issue.
In this study, we aim to detect HO2 using mid-infrared absorption spectroscopy and to elucidate its reaction. Mid-infrared light is expected to detect HO2 with high sensitivity because of its strong absorption. We have developed a new system for sensitive detection of HO2 using a novel laser source, quantum well laser.

the device
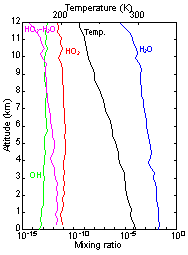
Predicted concentration of HO2-H2O complex
Concentration of HO2-H2O complex estimated from chemical reaction experiments
Predicted concentration of HO2-H2O complex
Elucidation of atmospheric chemistry and combustion chemistry mechanisms using high-sensitivity spectroscopy
We are studying the causes of photochemical smog, which has been gradually increasing in urban areas in recent years, from the standpoint of chemical reactions. The problems of urban atmospheric environment are shifting from industrial atmospheric problems to urban problems. In other words, the emission of chemical substances that we use in our daily lives into the atmosphere is considered to be the cause. In order to solve these problems, we are studying the chemical reactions that occur when chemical substances are released into the atmosphere from the standpoint of reaction kinetics.
Automotive fuels contain about 30-40% aromatic hydrocarbons. We are studying the atmospheric oxidation and combustion processes of aromatic hydrocarbons from both experimental and theoretical approaches. The figure below shows the oxidation process of aromatic hydrocarbons derived from automobile fuels.
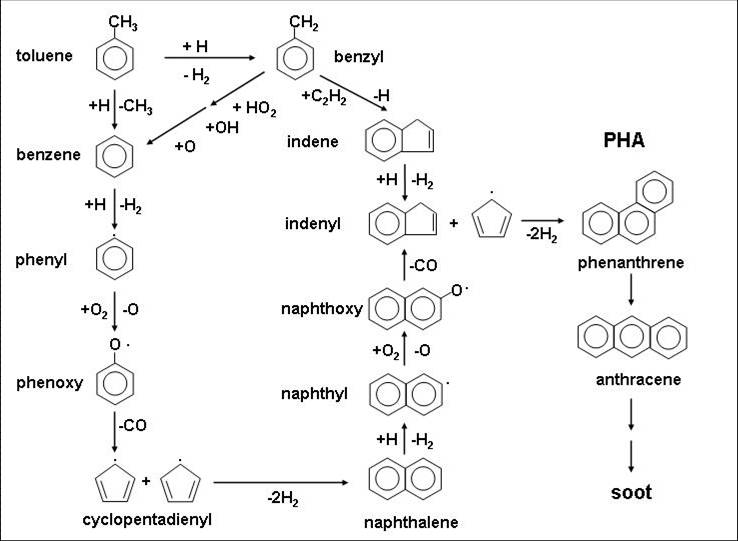
Oxidation process of aromatic hydrocarbons
Clarification of the mechanism of soot formation
Currently, diesel engines are attracting attention because they have a lower fuel consumption rate (i.e., lower carbon dioxide emissions) than spark-ignition engines in internal combustion engines (engines). However, soot, which is generated by incomplete combustion, is a major problem in diesel engines. DPF (Diesel Particulate Filter) is currently used as a method to reduce soot emissions. 90% of soot is trapped in DPF, which makes the exhaust gas clean, but it has a drawback that DPF needs to be replaced periodically.
However, DPFs have the disadvantage that they must be replaced periodically. Therefore, there is a need for a method to control the amount of soot production by controlling the combustion in diesel engines. In this study, we analyze soot using three analyzers (TOF-MS, SMPS, and ATOF-MS) in order to understand the combustion phenomena in diesel engines that affect the amount of soot produced, and to elucidate the mechanism of soot production.
Research on peat fires
Peatland stores about 1/3 of the carbon contained in all soils in the world, so the stability of peat has an important influence on climate change. Peat fires emit large amounts of particulate matter, carbon dioxide, and methane, causing international smoke pollution. Peat fires are prolonged and widespread because the combustion progresses from the ground surface to deep underground, making it difficult to extinguish. In recent years, a fire extinguishing agent for peat fires has been developed, mainly composed of alkali metal carbonates, which is mixed with water to accelerate penetration into the ground, cool the fire, inhibit pyrolysis and combustion reactions, and block oxygen. We are conducting research to understand the effects of these extinguishing agents on the exhaust gas from peat fires and their mechanisms.
Analysis of condensed phase reaction mechanisms for material safety
We are conducting experimental and computational analysis of condensed-phase reactions in order to improve the safety of handling reactive chemicals such as polymerizable substances, which have recently become a new concern for thermal hazards, based on our understanding of the phenomena. For chemical reactions involving large energy release in the condensed phase, such as thermal decomposition, polymerization, oxidation, etc., we use various analytical techniques to identify the chemical species produced and evaluate the hazards. We also perform quantum chemical calculations and reaction simulations that simulate the condensed phase in a purely theoretical manner, and compare them with experimental results to understand and control the phenomena at the elementary reaction level.
Journal from 2011 (with peer review)
2020
- Junting Qiu, Zhancong Liang, Kenichi Tonokura, Agustín J. Colussi, Shinichi Enami,”Stability of Monoterpene-Derived α-Hydroxyalkyl-Hydroperoxides in Aqueous Organic Media – Relevance to the Fate of Hydroperoxides in Aerosol Particle Phases”, Environ. Sci. Tech. accepted.
- Maya Minamida, Kotaro Tanaka, and Kenichi Tonokura, “Kinetic study of the oxidation reaction of 4-methylphenyl radical”, Int. J. Chem. Kinet. 52, 77-83 (2020).
2019
- Junting Qiu, Shinnosuke Ishizuka, Kenichi Tonokura, Agustín J. Colussi, Shinichi Enami, “Water Dramatically Accelerates the Decomposition of α‑Hydroxyalkyl-Hydroperoxides in Aerosol Particles”, J. Phys. Chem. Lett. 10, 5748-5755 (2019).
- Junting Qiu, Shinnosuke Ishizuka, Kenichi Tonokura, Kei Sato, Satoshi Inomata, Shinichi Enami, “Effects of pH on Interfacial Ozonolysis of α-Terpineol” , J. Phys. Chem. A, 123, 7148-7155 (2019).
- Kazuya Inoue, Kenichi Tonokura, Hiroyuki Yamada, “Modeling study on the spatial variation of the sensitivity of photochemical ozone concentrations and population exposure to VOC emission reductions in Japan”, Air Qual. Atmos. Health, 12, 1035-1047 (2019).
- Junting Qiu, Shinnosuke Ishizuka, Kenichi Tonokura, Shinichi Enami, “Interfacial vs Bulk Ozonolysis of Nerolidol”, Environ. Sci. Tech. 53, 5750-5757 (2019).
- Hiroo Hata, Koich Yanai, Masahiko Kugata, Hiroyuki Yamada, Genta Noumura, Kenichi Tonokura, “Modeling evaporative emissions from parked gasoline cars based on vehicle carbon canister experiments”, Sci. Total Environ. 675, 679-685 (2019)
- Hiroo Hata, Kenichi Tonokura, “Impact of next-generation vehicles on tropospheric ozone estimated by chemical transport model in the Kanto region of Japan”, Sci. Reports 9, 3573 (2019).
- Junting Qiu, Kenichi Tonokura, “Detection of the simplest Criegee intermediate CH2OO in the ν4 band using a continuous wave quantum cascade laser and its kinetics with SO2 and NO2”, Chem. Phys. Lett. X, 2, 100019 (2019).
- Yuta Matsumoto, Rumiko Hayashi, and Kenichi Tonokura, “Supercritical water oxidation as a pretreatment method for stable carbon isotope ratio analysis of rice”, J. Chem. Eng. Jpn. 52, 83-88 (2019).
2018
- Junting Qiu, Shinnosuke Ishizuka, Kenichi Tonokura, Agustín J. Colussi, and Shinichi Enami, “Reactivity of Monoterpene Criegee Intermediates at Gas–Liquid Interfaces”, J. Phys. Chem. A, 122, 7910-7917 (2018).
- Junting Qiu, Shinnosuke Ishizuka, Kenichi Tonokura, Shinichi Enami, “Reactions of Criegee Intermediates with Benzoic Acid at the Gas/Liquid Interface” , J. Phys. Chem. A, 122, 6303– 6310 (2018).
- Windy Iriana, Kenichi Tonokura, Gen Inoue, Masahiro Kawasaki, Osamu Kozan, Kazuki Fujimoto, Masafumi Ohashi, Isao Morino, Yu Someya, Ryuichi Imasu, Muhanmmad Arif Rahman, and Dodo Gunawan, “Ground-based measurement of column-averaged mixing ratios of carbon dioxide in the peatland fire-prone area of Central Kalimantan in Indonesia”, Sci. Reports, 8, 8437 (2018).
- Hiroyuki Yamada, Satoshi Inomata, Hiroshi Tanimoto, Hiroo Hata, and Kenichi Tonokura, “Estimation of refueling emissions based on theoretical model and effects of E10 Fuel on refueling and evaporative emission from gasoline cars”, Sci. Total Environ. 622-623, 467-473 (2018).
- Hiroo Hata, Hiroyuki Yamada, Kazuo Kokuryo, Megumi Okada, Chikage Funakubo, and Kenichi Tonokura, “Estimation model for evaporative emissions from gasoline vehicles on the basis of the theory of thermodynamics”, Sci. Total Environ. 618, 1685-1691 (2018).
2017
- Chikara Hashimoto, Kenichi Tonokura, “Development of a real-time measurement device of atmospheric carbon monoxide combined with mid-infrared wavelength modulation spectroscopy”, Chem. Lett. 46, 1501-1503 (2017).
- Kotaro Tanaka, Kai Miyamura, Kazushi Akishima, Kenichi Tonokura, and Mitsuru. Konno, “Measurement of ethylene in combustion exhaust using a 3.3-μm distributed feedback interband cascade laser with wavelength modulation spectroscopy”, Appl. Phys. B, 123, 219 (2017).
2016
- K. Tanaka, K. Miyamura, K. Akishima, K. Tonokura, and M. Konno, “Sensitive measurements of trace gas of formaldehyde using a mid-infrared laser spectrometer with a compact multi-pass cell”, Infrared Phys. Technol., 79, 1-5(2016).
- W. Iriana, K. Tonokura, M. Kawasaki, G. Inoue, K. Kusin, and S. H. Limin, “Measurement of carbon dioxide flux from tropical peatland in Indonesia by nocturnal temperature-inversion trap method”, Environ. Res. Lett., 11, 095011 (2016).
- Y. Tsuji, K. Tonokura, R. Hayashi, “Chemical substances management system at the University of Tokyo”, J. Environ. Safety, 7, 129-131 (2016).
- H. Yamada, R. Hayashi, K. Tonokura, “On-road NO2 and particle number concentrations regarding their potential impacts on in-vehicle air qualities”, Sci. Total Environ., 563-564, 944-955 (2016).
- K. Funasaka, D. Asakawa, Y. Oku, N. Kishikawa, Y. Deguchi, N. Sera, T. Seiyama, K. Horasaki, K. Arashidani, A. Toriba, K. Hayakawa, M. Watanabe, H. Kataoka, T. Yamaguchi, F. Ikemori, Y. Inaba, K. Tonokura, M. Akiyama, O. Kokunai, S. Coulibaly, Tomohiro Hasei, T. Watanabe, “Spatial correlativity of atmospheric particulate components simultaneously collected in Japan.”, Environ. Monit. Assess., 188, 85 (2016).
- K. Tonokura and R. Takahashi, “Pressure broadening of the ν1 band of nitrous oxide by carbon dioxide”, Chem. Lett., 45, 95-97 (2016).
2015
- T. Kinoshita, K. Tonokura, I. Shibata, E. Yamada, S. Murata, M. Matsumoto, M. Sawamura, H. Nakagawa, K. Morimoto, and A. Koyama, “Management of chemicals for safety and education in laboratory”, J. Environ. Safety, 6, 81-84 (2015).
2014
- M. Minamida, K. Tonokura, “Measurements of Air Broadening Coefficients of Hydroperoxyl Radical in the ν3 band”, J. Quant. Spectrosc. Radiat. Transfer, 148, 65-69 (2014).
- X.Guo, T. Nakayama, H. Yamada, S. Inomata, K. Tonokura, Y. Matsumi, “Measurements of light absorbing properties of diesel exhaust particles using a three wavelength photoacoustic spectrometer”, Atmos. Environ. 94, 428-437 (2014).
- K. Tanaka, K. Takahashi, K. Tonokura, H. Sugiyama, N. Nakano, and Y. Nakano, “Detection of Stable Carbon Isotopes of Methane with a 2.4μm Distributed Feedback Laser”, J. Quant. Spectrosc. Radiat. Transfer, 133, 670-674 (2014).
2013
- K. Tanaka,R. Kojima, K. Takahashi, and K. Tonokura, “Continuous measurements of stable carbon isotopes in CO2with a near-IR laser absorption spectrometer”,Infrared Phys. Technol. 60, 281-287 (2013).
- Y. Sakamoto, S. Enami, K. Tonokura, “Enhancement of gaseous iodine emission by aqueous ferrous ions during the heterogeneous reaction of gaseous ozone with aqueous iodide”,J. Phys. Chem. A, 117, 2980-2986 (2013).
2012
- Y. Yamamoto, Y. Kambe, H. Yamada, and K. Tonokura, “Measurement of Volatile Organic Compounds in Vehicle Exhaust Using Single Photon Ionization Time-of-Flight Mass Spectrometry”,Anal. Sci. 28, 385-390 (2012).
- Y. Kambe, Y. Yamamoto, H. Yamada, and K. Tonokura, “Measurement of Gas- and Particle-Phase Organic Species in Diesel Exhaust Using Vacuum Ultraviolet Single Photon Ionization Time-of-Flight Mass Spectrometry”,Chem. Lett. 41, 292-294 (2012).
- Y. Kambe, Y. Yoshii, K. Takahashi, and K. Tonokura, “Monitoring of atmospheric nitrogen dioxide by long-path pulsed differential optical absorption spectroscopy using two different light paths”,J. Env. Monitor. 14, 944-950 (2012).
- Y. Sakamoto, K. Tonokura, “Measurements of the absorption line strength of hydroperoxyl radical in the n3 band using a continuous wave quantum cascade laser”, J. Phys. Chem. A, 116, 215-222 (2012).
- K. Tanaka, M. Ando, Y. Sakamoto, and K. Tonokura, “Pressure dependence of phenylperoxy radical formation in the reaction of phenyl radical with molecular oxygen”, Int. J. Chem. Kinet. 44, 41-50 (2012).
2011
- Y. Yamamoto, H. Sumizawa, H. Yamada, and K. Tonokura, “Real-time measurement of nitrogen dioxide in vehicle exhaust gas by mid-infrared cavity ring-down spectroscopy”, Appl. Phys. B 105, 923-931(2011).
- K. Tanaka and K. Tonokura, “Sensitive measurements of stable carbon isotopes of CO2 with wavelength modulation spectroscopy near 2 mm ”, Appl. Phys. B 105, 463-469 (2011). (selected as a research highlight in Nature Photonics)
- H. Suzuki, Y. Miyao, T. Nakayama, J. K. Pearce, Y. Matsumi, K. Takahashi, K. Kita, and K. Tonokura, ”Comparison of laser-induced fluorescence and chemiluminescence measurements of NO2 at an urban site”, Atoms. Environ. 45, 6233-6240 (2011).
- Y. Sakamoto, K. Tanaka, T. Asakawa, and K. Tonokura, “Wavelength modulation spectroscopy detection of N2O using mid-infrared laser from a direct-bonded quasi-phase-matched LiNbO3 ridge waveguide”, Jpn. J. Appl. Phys. 50, 062401 (2011).
- J.-H. Xing, K. Takahashi, A. Yabushita, T. Kinugawa, T. Nakayama, Y. Matsumi, K. Tonokura, A.Takami, T. Imamura, M. Kawasaki, T. Hikida, and A. Shimono, “Characterization of aerosol particles observed in the Tokyo metropolitan area in summer 2008 by two different particle mass spectrometers”, Aerosol Sci. Tech. 45, 315 (2011).
- S. Miyano and K. Tonokura, “Mid-infrared Spectroscopy in the n3 band of the hydroperoxyl radical using a continuous wave quantum cascade laser”, J. Mol. Spectrosc. 264, 47 (2011).
